Included Content
- Chemistry Review I & II
- Overview of Metabolism I & II
- Macromolecules I (Proteins)
- Hemoglobin & Myoglobin: Structure, function, & acid-base relationships in respiration
- Enzyme & Enzyme Kinetics I & II
- Macromolecules II (Carbohydrates)
- Macromolecules III (Nucleic Acids)
- Macromolecules IV (Lipids & Lipid Metabolism)
- Biomembranes & Membrane Transport
- Cell Signaling I & II
- Cell Signaling TBL
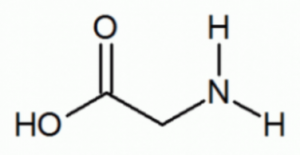
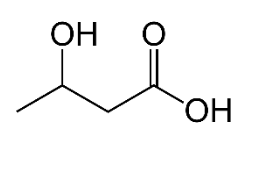
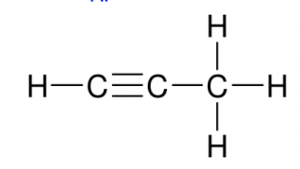
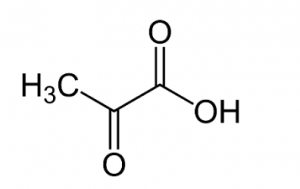
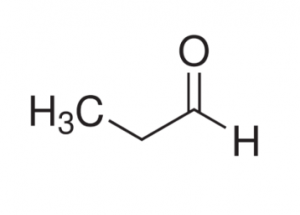
- Identify functional groups in the given compound:

- Amide & carboxylic acid
- Ester & Amine
- Amine & Carboxylic acid
- Amide & Aldehyde
- Aldehyde & AmineAnswer C.
Chemistry Review.
What is the mole fraction of sulfuric acid in a solution made by adding 3.4 grams of sulfuric acid (98.1 g/mol) to 3,500 mL of water (18 g/mol)?
- 1.8 x 10-4
- 9.98 x 10-2
- 3.8 x 10-3
- 1.4 x 10-4Answer A.
#moles of H2SO4: 3.5 g ÷ 98.1 g/mol = 0.3568… mol
#moles of H2O: 3500 mL = 3500 g ÷ 18 g/mol = 194 mol
Mole Fraction = #moles H2SO4 ÷ (#moles H2SO4 + #moles H2O)= 0.03568 ÷ 194 = 1.83 x 10-4
Chemistry Review.
Which of the following is represents a ketone body?
Ketone bodies have an important biological function in providing energy in the later stages of a fast. Which of the following is INCORRECT?
- Liver cannot metabolize ketone bodies
- Ketone bodies can be utilized by skeletal muscles
- Ketone bodies can not be used as substrate for central nervous system
- Our body is well adapted to handle very high levels of ketone bodies
- Ketone bodies are soluble compounds derived from fatty acids.Answer D. High levels of ketone bodies can lead to ketoacidosis.
Chemistry Review.
An excess production of ketone bodies is often present in those in a fasting state or with untreated type I diabetes. What mechanism would most likely produce conditions for increased ketone body production?
- HMG CoA lyase deficiency
- High malonyl CoA concentration
- High levels of ATP feeding forward in the FA production pathway
- Low blood insulin levels
- Low blood glucagon levelsAnswer D.
Chemistry Review.
The density of ethanol is 0.789 g/mL. How many grams of ethanol (46 g/mol) should be mixed with 225 mL of water to make a 4.5% (v/v) mixture?
- 2.5 x 10-4 g
- 7.99 g
- 1.25 x 10-1 g
- 4.54 gAnswer B.
4.5% x 225 mL = 10.125 mL EtOH
0.789g/mL • 10.125 mL EtOH = 7.99 g
Chemistry Review.
What is the pH of a 0.275 M hydrochloric acid solution. Ka = 3.0 x 10-8?
- 3.03
- 4.04
- 5.05
- 2.02Answer B.
pH = -log[H+]
HCl → H+ + Cl-
([Cl- ][H+])/[HCl] = 3.0 x 10-8
X2/(0.275 M) = 3.0 x 10-8
-log(X) = 4.04)
Chemistry Review.
What is the molarity of 0.1115 N Sr(OH)2?
- 0.1115 M
- 0.0558 M
- 0.0372 M
- 0.230 MAnswer B.
Normality = molarity x Eq.
Ions will dissociate: Sr2+ | (OH-)2
1 x |+2| = 2
|-1| x 2 = 2
Either way you calculate it, Eq is 2.
0.1115 N ÷ 2 = 0.0558 M
Chemistry Review.
What is the normality of 0.321 g sodium carbonate (Na2CO3, 106 g/mol) in 250.0 mL of solution?
- 0.0242N
- 0.0726N
- 0.0484N
- 0.0161NAnswer A.
Normality = mol • #EqVolume
Eq = charge of Sodium Ion x 2 = (+1) x 2 = 2
(0.321 g)/(106 g/mol)= .003028 mol Na2CO3
(0.003028 mol x 2 Eq)/(0.250 L )
(0.003028 mol • 2 Eq)/0..250 L = 0.0242 N
Chemistry Review.
Identify the most reduced molecule:
Which of the following is NOT a reducing agent?
- NAD+
- FADH2
- NADH/H+
- FMH2Answer A.
Overview of Metabolism.
Which of the following is NOT a one carbon carrier?
- Biotin
- SAM (S-adenosylmethionine)
- Tetrahydrofolate
- NiacinAnswer D.
Overview of Metabolism.
Which of the following does NOT use substrate level phosphorylation as a mechanism to create ATP?
- Glycolysis
- Pyruvate production
- Citric Acid Cycle
- Electron Transport ChainAnswer D.
Overview of Metabolism.
- Which of the following vitamins is/are vital for the production of Acetyl CoA from Pyruvate?
- Vitamin B1
- Vitamin B12
- Vitamin B3
- A & B
- A & CAnswer E.
Vitamin B3 (Niacin) is responsible for the production of NAD+, which is a necessary oxidizing agent used in the reaction of Pyruvate to Acetyl-CoA
Vitamin B1 (Thiamine) is a coenzyme for Pyruvate dehydrogenase (PDH), the enzyme that converts Pyruvate to Acetyl-CoA.
Although not an answer choice, Vitamin B5 (Pantothenic Acid) is also vital to the formation of Acetyl-CoA since it is a necessary cofactor for CoA.
B Vitamins: First Aid USMLE Step 1 (2019), pgs. 66-69.
Overview of Metabolism.
Which of the following are necessary for the catabolism of fatty acids?
- Pantothenic Acid (Vitamin B5)
- Acetyl-CoA
- Biotin (Vitamin B7)
- NADPH
- All of the aboveAnswer E.
Overview of Metabolism.
Which of the following cofactors serves a function in the rate-limiting carboxylation step of fatty acid synthesis?
- Niacin
- Cobalamin
- Thiamine
- Biotin
- Pantothenic acidAnswer D.
Overview of Metabolism.
Acetyl CoA is metabolic product of which of the following?
- Amino Acids
- Fatty Acids
- Glucose
- B & C
- A, B & CAnswer E.
Overview of Metabolism.
Which of the following is an oxidative pathway?
- Catabolism
- Anabolism
- Glycolysis
- A & C
- B & CAnswer D.
Overview of Metabolism.
Tetrahydrofolate is involved in which of the following pathways?
- Purine production
- Pyrimidine Production
- Amino Acid production
- A & C
- A, B & CAnswer E. Overview of Metabolism.
Which of the following condition(s) would present as B12 deficiency?
- excessive use of methotrexate
- Deficiency of Intrinsic factors
- Deficiency of Folate
- A & B
- A, B & CAnswer E.
Overview of Metabolism.
Which of the following is INCORRECT regarding prions?
- They are infectious proteins that result from the misfolding of native proteins
- CJD, Scrapie, and Kuru are all examples of infectious proteins
- Prions can stay dormant for years before causing a disease state
- They are easily treated with gene therapyAnswer D. There is no known cure for diseases caused by prions. All other choices are correct.
Macromolecules (Proteins).
Velcade, otherwise known as Bortezomib, is an anti-cancer drug used to treat multiple myeloma and mantle cell lymphoma. It works by binding to proteasomes leading to their inactivation. Which of the following would best explain the mechanism of action of this drug?
- Velcade prevents degradation of extracellular proteins that are responsible for tagging cancer cells for cell death
- Velcade prevents degradation of intracellular pro-apoptotic proteins
- Velcade causes an increase in ubiquitin production, tagging more proteins for degradation
- Velcade inhibits Cathepsin K, inhibiting degradation of extracellular proteins
- None of the aboveAnswer B. The ubiquitin-proteasome pathway is responsible for the intracellular degradation of proteins. Therefore, answer choices A & D are out. While ubiquitin is responsible for tagging proteins for proteasome degradation, the question states that Velcade inhibits the proteasome. So this mechanism doesn’t make sense as it pertains to the drugs ability to treat cancer. Answer B is the best choice because it is concerning intracellular pro-apoptotic factors, which are responsible for initiating cell death.
Macromolecules (Proteins).
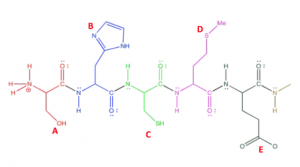
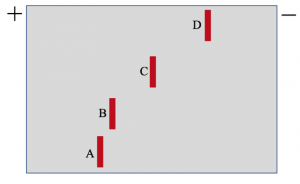
The picture below shows a polypeptide chain with numerous amino acids. Which of the following would most likely undergo the post-translational modification of phosphorylation?

- A
- B
- C
- D
- EAnswer A. Phosphorylation occurs on amino acids with hydroxyl groups (i.e. serine, threonine, and tyrosine). Option A is serine.
Macromolecules (Proteins).
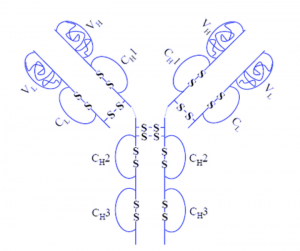
- Adalimumab, also called Humira, is an injectable Immunosuppressive drug that can treat arthritis, plaque psoriasis, and Crohn’s disease. The structure of Humira is shown below. Which of the following best describes the reasoning behind the administration of Humira as an injectable as opposed to other routes (i.e. orally)?

- The low pH of the stomach would break the disulfide bonds
- The low pH of the stomach would cause breakage of salt bridges present on protein subunits
- The stomach acid would cause early activation of the drug
- The low pH would cause cleavage in the peptide bonds
- A & BAnswer B. This one’s kind of tricky, especially if you are relying on the picture to answer the question. Because disulfide bonds are covalent, they will not be broken by the low pH of stomach acid. The only way these bonds would break was if there was a reducing agent in the stomach, which wasn’t an answer choice.
Macromolecules (Proteins).
Pycnodysostosis is an autosomal recessive condition caused by mutations in the gene that codes the enzyme cathepsin K (CTSK) on chromosome 1q21. Which of the following would be a result from this disease?
- Inability to degrade endogenous proteins
- Inability to degrade exogenous proteins
- Inability to tag proteins with Ubiquitin and target them for degradation
- A & C
- B & CAnswer B. Cathepsin K is vital for lysosomal degradation of proteins which only targets exogenous proteins (extracellular). Answers A and C would be concerned with the ubiquitin-proteasome pathway which is the degradation of endogenous proteins.
Macromolecules (Proteins).
Which of the following is an example of a cofactor?
- Heme
- NAD+
- FAD+
- Zn2+
- All of the aboveAnswer E. A cofactor is defined as an inorganic or organic molecule required for protein activity. Heme is a prosthetic group on Hemoglobin, NAD and FAD are coenzymes, and Zinc in a cofactor. However, coenzymes and prosthetic groups are by definition specified forms of cofactors. Therefore, all of the mentioned molecules are technically cofactors. Macromolecules (Proteins).
Which of the following is TRUE regarding lysosomal degradation of proteins?
- It is concerned with exogenous proteins
- It is concerned with endogenous proteins
- It is due to Cathepsin family of cysteine proteases
- A & C
- B & CAnswer D.
Macromolecules (Proteins).
Which of the following is true regarding peptide bonds?
- They form from dehydration reactions between amino acids
- They form from condensation reactions between amino acids
- It is a bond between the alpha carbon and the N terminus of two amino acids
- A & B
- A, B, & CAnswer B. Peptide bonds are the result of condensation reactions (also called dehydration reactions) between the C terminus and the N terminus of two amino acids. The alpha carbon is the carbon with the side chain, which is not involved in the peptide bond.
Macromolecules (Proteins).
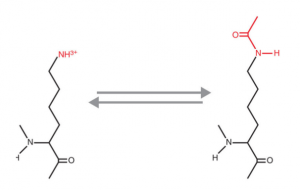
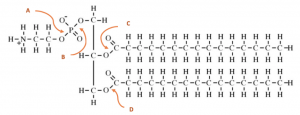
The picture below is an example of what post-translational modification

- Hydroxylation
- Phosphorylation
- Methylation
- SUMOylation
- AcetylationAnswer E. Acetylation results from the addition of an acetyl group to a Lysine residue or the N-terminus of a protein. The above example shows acetylation of a Lysine residue.
Macromolecules (Proteins).
Carbon monoxide combines with hemoglobin to produce carboxyhemoglobin, which usurps the space in hemoglobin that normally carries oxygen, but is ineffective for delivering oxygen to bodily tissues. This can inhibit the electron transport chain, and subsequently, the process of oxidative phosphorylation. Which of the following would demonstrate a typical ABG for an individual with CO poisoning?
- pH = 7.26, pCO2= 34, pO2= 100, HCO3-= 15
- pH = 7.35, pCO2= 45, pO2= 100, HCO3-= 15
- pH = 7.45, pCO2= 36, pO2= 89, HCO3-= 23
- pH = 7.26, pCO2= 45, pO2= 98, HCO3-= 23
- pH = 7.45, pCO2= 34, pO2= 68, HCO3-= 15Answer A. Since CO poisoning inhibits OXPHOS, it will cause a buildup of lactate in the body, causing metabolic acidosis. Metabolic acidosis is usually associated with a low pH and low bicarbonate, which is show in answer A. The body compensates for this by hyperventilating, accounting for the low pCO2. There is a normal PO2 because the carbon monoxide concentration required to cause poisoning is low enough that it does not alter the amount of oxygen physically dissolved in the plasma, only the ability of Hb to release the oxygen to the tissues.
Hemoglobin & Myoglobin Structure, Function, and Acid-base Relationships in Respiration.
Opioids affect the part of the brain that regulates breathing. When people take high doses of opioids, it can lead to an overdose, with the slowing or stopping of breathing and sometimes death. Based on this information, a patient who has overdosed on opioids would be exhibiting:
- Respiratory alkalosis
- Respiratory acidosis
- Metabolic acidosis
- Metabolic alkalosis
- HypokalemiaAnswer B. Opioids decrease breathing, leading to hypoventilation. Hypoventilation causes a buildup of CO2 in the body and associated respiratory acidosis.
Hemoglobin & Myoglobin Structure, Function, and Acid-base Relationships in Respiration.
A patient arrives with headache, warm/flushed skin, decreased BP, hyperventilation, nausea, vomiting, and diarrhea. If you order a blood test, what do you expect to find?
- Blood K+ levels are high, pH is low
- Blood K+ levels are low, pH is low
- Blood K+ levels are high, pH is high
- Blood K+ levels are low, pH is high Answer A. The patient is showing signs of metabolic acidosis. The dead giveaway is the hyperventilation coupled with vomiting. In acidosis, blood levels of potassium are high. This is thought to be caused by a cellular mechanism in which H+ and K+ are exchanged by the cell in order to raise the pH of the blood. Acidosis implies low pH, and is accompanied by high levels of K+ in plasma. Important side note: Diarhea causes loss of HCO3- . This patient is probably suffering acidosis because of the diarrhea, and then the other symptoms are setting in.
Hemoglobin & Myoglobin Structure, Function, and Acid-base Relationships in Respiration.
COPD is a disease that causes inflammation in the bronchioles, leading to blockage which impedes air flow in and out of the lungs. As a result, patients are unable to rid their body of CO2. The administration of O2 is a common treatment option for patients exhibiting COPD. However, prolonged treatment with oxygen can decrease CO2 transport in the blood and cause pCO2 to elevate even further. Which of the following terms best describes this effect?
- Bohr Effect
- Haldane Effect
- Metabolic alkalosis
- Respiratory alkalosis
- None of the above Answer B. The Haldane effect describes the process by which O2 affects Hb’s affinity for CO2. Deoxygenation of Hb causes an increased CO2 transport to lungs, while oxygenation of Hb would cause decreased CO2 transport to lungs. The Bohr effect conversely describes the process by which CO2 and H+ affect the ability of Hb to bind to O2. Since oxygen administration is the treatment described, this effect doesn’t quite describe the process at play. The others just don’t make sense.
Hemoglobin and Myoglobin: Structure, Function and Acid-base Relationships in Respiration.
A 32 y/o African American male patient presents to the ED with symptoms including muscle pain, weakness, vomiting, and confusion. He was involved in an MVA where his right leg became crushed under the dashboard. He was trapped in his vehicle for approximately 2 hours before anyone was able to find him and call 911. You notice a brown tint to his urine. What is most likely the cause for the patient’s symptoms?
- Pyruvate kinase deficiency
- Sickle cell disease
- Methemoglobinemia
- Rhabdomyolysis
- Metabolic acidosis Answer D. Rhabdomyolysis is a condition in which skeletal muscle breaks down rapidly, causing a release of Mb into the blood stream. It is often the result of strenuous exercise or crush injuries. As Mb is released into the blood stream, the body attempts to excrete it through the urine causing it to turn brown.
Hemoglobin and Myoglobin: Structure, Function and Acid-base Relationships in Respiration.
Carbon monoxide combines with hemoglobin to produce carboxyhemoglobin, which usurps the space in hemoglobin that normally carries oxygen, but is ineffective for delivering oxygen to bodily tissues. This can inhibit the electron transport chain, and subsequently, the process of oxidative phosphorylation. Which of the following would demonstrate a typical ABG for an individual with CO poisoning?
- pH = 7.26, pCO2= 34, pO2= 100, HCO3-= 15
- pH = 7.35, pCO2= 45, pO2= 100, HCO3-= 15
- pH = 7.45, pCO2= 36, pO2= 89, HCO3-= 23
- pH = 7.26, pCO2= 45, pO2= 98, HCO3-= 23
- pH = 7.45, pCO2= 34, pO2= 68, HCO3-= 15 Answer A. Since CO poisoning inhibits OXPHOS, it will cause a buildup of lactate in the body, causing metabolic acidosis. Metabolic acidosis is usually associated with a low pH and low bicarbonate, which is show in answer A. The body compensates for this by hyperventilating, accounting for the low pCO2. There is a normal PO2 because the carbon monoxide concentration required to cause poisoning is low enough that it does not alter the amount of oxygen physically dissolved in the plasma, only the ability of Hb to release the oxygen to the tissues.
Hemoglobin & Myoglobin Structure, Function, and Acid-base Relationships in Respiration.
An inability of Hb to release O2 to tissues could be a result of which of the following
- A deficiency in bisphosphoglycerate mutase
- A deficiency in Pyruvate kinase
- A deficiency in 2,3-BPG
- A & C
- A, B, & CAnswer D. 2,3-BPG is responsible for assisting Hb in releasing the O2 to Mb once it reaches the tissues. Likewise, a deficiency in bisphosphoglycerate mutase would cause a decrease in 2,3-BPG since it is responsible for facilitating the reaction of 1,3-BPG –> 2,3-BPG. A deficiency in Pyruvate kinase would cause Methemoglobinemia and would affect the ability of Hb to bind to O2, not release it.
Hemoglobin & Myoglobin Structure, Function, and Acid-base Relationships in Respiration.
Which of the following is NOT true regarding the reaction facilitated by Hexokinase?
- Hexokinase follows the induced fit model
- Hexokinase catalyzes the reaction of glucose to glucose-6-phosphate in glycolysis
- Mg2+ is a cofactor involved in the reaction
- ATP is a co-substrate involved in the reaction
- The active site of Hexokinase allows water to act as a nucleophileAnswer E.
Enzymes and Enzyme Kinetics.
Which of the following is TRUE regarding Competitive inhibitors?
- They are often transition state analogs
- They are often substrate analogs
- They decrease the Km
- A & B
- B & CAnswer D. Competitive inhibitors can be either transition state analogs or substrate analogs. They work by binding to the active site of enzymes, increasing the Km (decreasing affinity). They do not change the Vmax.
Enzymes and Enzyme Kinetics.
Glucokinase is a similar enzyme to hexokinase in that it catalyzes the reaction of Glucose to Glucose-6- phosphate. However, glucokinase is different to hexokinase in that glucokinase…
- has a greater Km and lower affinity for glucose
- has a lower Km and greater affinity for glucose
- Glucokinase is located in the liver
- A & C
- B & CAnswer D. Glucokinase is present in the liver and pancreas. It has a lower affinity for Glucose (compared to hexokinase), and thus a higher Km.
Enzymes and Enzyme Kinetics.
Atropine is a medication used to treat organophosphate poisonings, usually in conjunction with other drugs such as pralidoxime. It works by selectively blocking the binding of the neurotransmitter acetylcholine to its receptor in nerve cells. Which of the following is true regarding Atropine?
- Atropine blocks ACh from binding to cholinergic receptors
- Atropine prevents the “aging” of organophosphates
- Atropine is a competitive inhibitor for AChE
- A & B
- A, B, & CAnswer A. The question states two key facts about Atropine that should help you. The first is that Atropine is often used in conjunction with pralidoxime, which we know treats insecticide poisoning by preventing the aging of organophosphates. If we know that Atropine is used in conjunction with these drugs, we should be able to infer that it doesn’t have the same function. Therefore C & E are out. The second fact is that Atropine works by blocking ACh from its receptors (which are specifically cholinergic receptors). Answer C doesn’t make sense because if Atropine was a competitive inhibitor for AChE, it would have a similar effect that organophosphates have.
Enzymes and Enzyme Kinetics.
Which of the following would NOT be considered an Acetylcholine esterase (AChE) inhibitor?
- Neostigmine
- Pyriodostigmine
- Physiotigmine
- Pralidoxime
- AriceptAnswer D. Neostigmine, Pyrdostigmine, and Physiotigmine are all form of Carbamates with pharmacological purposes. Aricept is an AChE inhibitor that treats dementia. Pralidoxime is the only answer that is not an AChE inhibitor, but actually works to reverse the effects of organophosphate poisoning.
Enzymes and Enzyme Kinetics.
Which of the following is NOT true regarding a Line-Weaver Burk plot?
- The x-intercept represents -1/Km
- The y-intercept represents 1/Vmax
- A competitive inhibitor would have a greater slope than an enzyme without inhibition
- A noncompetitive inhibitor would have a greater slope than an enzyme without inhibitionAnswer D.
Enzymes and Enzyme Kinetics.
Which of the following is NOT true regarding Acetylcholinesterase (AChE)?
- The active site consists of a catalytic triad composed of Lys, Ser, & His
- His 440 works as an acid catalyst in the reaction
- His 440 works as a base catalyst in the reaction
- Ser 200 functions by binding to the acetyl group on ACh
- The anionic subsite accommodates the positive quaternary amine of AChAnswer A. The active site consists of a catalytic triad composed of Glu, Ser, & His.
Enzyme and Enzyme Kinetics.
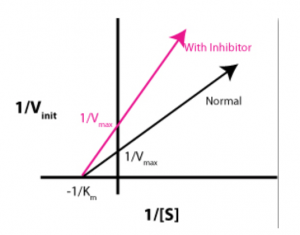
Lineweaver-Burk plots can be used to visualize the effect that regulatory molecules have on enzyme kinetics. The graph below demonstrates which type of regulator?

- Competitive inhibitor
- Non-competitive inhibitor
- Un-competitive inhibitor
- Allosteric activator
- Mixed inhibitorAnswer B. Noncompetitive inhibitors affect the Vmax of the enzyme, but have no effect on Km. Uncompetitive inhibitors and competitive inhibitors both change Km, so (A) and (D) can be eliminated. Suicide inhibitors, or irreversible inhibitors, covalently modify the active site and destroy the function of the enzyme. Allosteric activator could be a good distractor for this question because the slope of the “with inhibitor” line is higher. However, Lineweaver-Burk plots are double reciprocal plots, so an increased slope means a lower Vmax.
Enzymes and Enzyme Kinetics.
A patient comes into the ED after overdosing on Tylenol (acetaminophen). He mentions that he was depressed after losing the OMS-I presidential election, and that he had been drinking as well. Which of the following is true regarding this patient’s ability to metabolize the Tylenol?
- Glutathione levels decrease with alcohol consumption
- Cytochrome P450 has increased causing NAPQI levels to rise
- GSH conjugation of NAPQI has increased
- A & C
- A & BAnswer E.
Enzyme and Enzyme Kinetics.
The following reaction is catalyzed by which of the following enzymes?

- Xanthine oxidase
- Hypoxanthanine-guanine phosphoribosyl transferase (HGPRT)
- Ribonucleotide reductase
- Adenine phosphoribosyl transferase (APRT)
- PhosphofructokinaseAnswer C. A ribonucleotide is being reduced.
Enzymes and Enzyme Kinetics.
Which of the following is a allosteric inhibitor of phosphofructokinase (PFK1)?
- ATP
- AMP
- PRPP
- ADP
- Fructose-2,6-bisphosphateAnswer A.
Enzymes and Enzyme Kinetics.
Which of the following would be an indication that a myocardial infarction (MI) has occurred in a patient?
- Creatine Kinase BB will be high
- Creatine Kinase MM will be high
- Creatine Kinase MB will be high
- Decreased levels of PFK-1
- All of the aboveAnswer C.
Enzymes and Enzyme Kinetics.
- The picture below shows a reaction that is most likely catalyzed by which class of enzyme?
- Transferase
- Oxidoreductase
- Ligase
- Isomerase
- HydrolaseAnswer D.
Enzyme and Enzyme Kinetics.
Diabetes Mellitus (DM) is a group of diseases that result in hyperglycemia (too much sugar in the blood). In DM Type II, one would develop a decreased ability to react to insulin levels. Base on your knowledge regarding glucose transporters, which transporter is most likely affected by DM Type II?
- SGLT
- GLUT1
- GLUT2
- GLUT3
- GLUT4Answer E. GLUT4 transporters are located in skeletal muscle and adipose tissue and are responsive to insulin. They have a low Km and a high affinity for glucose. Since DM Type II results in a decreased sensitivity to insulin, GLUT4 transporters must be affected.
Macromolecules (Carbohydrates).
Which of the following is true regarding digestion of carbohydrates?
- α-amylase cleaves α-1,4 linkages in the stomach to produce Dextrins
- Sucrase cleaves α-1,2 linkages in the small intestine to produce glucose and fructose
- Fiber is absorbed by small intestine parietal cells
- Pancreatic α-amylase cleaves α-1,6 linkages to produce Disaccharides
- Maltase-Isomaltase cleaves α-1,6 and α-1,4 linkages in the small intestine to produce galactose and glucoseAnswer B.
Macromolecules (Carbohydrates).
Which of the following is NOT true regarding our ability to digest complex carbohydrates?
- Amylose can be broken down by α-amylase
- Amylopectin can be broken down by α-amylase
- Cellulose cannot be broken down since we lack enzymes that can hydrolyze ß-1,4 linkages
- Hemicellulose is considered a soluble fiberAnswer D. Hemicellulose is an insoluble fiber because (like cellulose) it has α-1,4 linkages that we cannot break down. Pectins and gums are considered to be soluble fibers.
Macromolecules (Carbohydrates).
A patient that is lactose intolerant will most likely be unable to…
- Hydrolyze α-1,6 linkages
- Hydrolyze β-1,4 linkages
- Hydrolyze α-1,2 linkages
- Hydrolyze α-1,4 linkages
- Both A & DAnswer B. Lactase hydrolyzes β-1,4 linkages to produces galactose and glucose in the small intestine.
Macromolecules (carbohydrates).
Individuals with De Vivo Disease (GLUT1 deficiency syndrome) present with several health issues pertaining mostly to their body’s decreased ability to transport glucose to which type of cells?
- The neurons of the brain
- Pancreatic beta cells
- Skeletal muscle
- Glial cells of the blood brain barrier
- Sperm cellsAnswer D. GLUT1 is the primary transporter for glucose in cells serving a barrier function, such as the supporting glial cells forming the blood-brain barrier. Pancreatic beta cells and skeletal muscle cells transport glucose using GLUT2 and GLUT 4, respectively. While the problem in those with De Vivo Disease is getting the necessary volume of glucose to the brain, the neural cells themselves have correctly functioning GLUT3, so (A) is not the best answer for this question.
Macromolecules (Carbohydrates).
Which of the following would be most beneficial in determining the effectiveness of treatment for a patient with diabetes mellitus?
- Determining fasting BGL
- Determine level of Hbf
- Determine level of HbA1c
- Determine level of HbSAnswer C.
Macromolecules (Carbohydrates).
Which of the following is NOT true of glycogen?
- It contains both α-1-4 and α-1-6 glyosidic bonds
- It has a higher osmotic effect than equivalent unbranched glucose
- It contains both reducing and non-reducing sites
- It can be broken down at multiple sites simultaneously
- An increase in insulin would cause an increase in glycogen productionAnswer B.
Macromolecules (Carbohydrates).
- Fehling’s reaction can be used to indicate the presence of sugars in the urine, creating a red precipitate when interacting with any reducing sugar. Which of the following common sugars would go undetected by this reaction?
- Fructose
- Glucose
- Sucrose
- Galactose
- LactoseAnswer C. Fehling’s reaction tests for reducing sugars. Sucrose is a non-reducing sugar.
Macromolecules (Carbohydrates).
Sickle Cell anemia is most often the result of the E6V mutation in HbS. However, it can also result from an E6K forming HbC. Based on what you know regarding isoelectric focusing and Hb variants, which of the following correctly pairs the variation of Hb with those shown on the electrophoresis at physiologic pH?

- A: HbA, B: HbF, C: HbS, D: HbC
- A: HbF, B: HbA, C: HbC, D: HbS
- A: HbF, B: HbA, C: HbS, D: HbC
- A: HbA, B: HbF, C: HbC, D: HbS
- A: HbS, B: HbC, C: HbF, D: HbAAnswer A. Hemoglobin electrophoresis is utilized to asses variations of hemoglobin. HbS (Sickle Cell) is caused when a missence mutation occurs allowing for the substitution of glutamate (negatively charged amino acid) with that of valine (a non polar amino acid). HbC has a glutamate residue replaced by Lysine, and thus a more positive charge than HbS. In Fetal Hemoglobin (HbF) histidine is replaced with serine disallowing interaction with 2,3-BPG and giving it greater affinity to oxygen.
Sickle Cell Anemia.
See “missence mutation” in First Aid USMLE Step 1 (2019), Pg. 38.
A patient with Sickle Cell Anemia comes into your office, his nerdy friend who is obsessed with cell physiology asks you the significance of this disease with regard to membrane potentials. Which of the following is the best answer?
- Since sickle cells are less negatively charged than normal cells, other cell membranes depolarize.
- Since sickle cells are more negatively charged than normal cells, other cell membranes hyperpolarize.
- Since sickle cells carry less O2, cell membranes depolarize.
- Since sickle cells carry more O2, cell membranes hyperpolarize.Answer C. Since sickle cells carry less O2 than normal cells (because O2 can’t access the binding sights as easily), less OXPHOS occurs, lowering ATP. Lowering ATP lowers activity of K+Na+ATPase. ATPase is electrogenic, and constantly makes the cell’s interior more negative. Thus, when it functions less (because there is less ATP) positive charge builds up in the cell, depolarizing the membrane.
Sickle Cell Anemia TBL.
Which of the following would NOT result in a decreased supply of nucleic acids for DNA replication?
- Introduction of methotrexate
- 5-fluorouracil
- 6-mercaptopurine
- Allopurinol
- Decreased expression of OMP CarboxylaseAnswer D. allopurinol limits purine degradation by blocking the oxidation of xanthine to uric acid, increasing salvage of these bases. Methotrexate limits the pool of available FH4, limiting dTMP production. 5-fluorouracil also prevents dTMP synthesis by irreversibly binding thymidylate synthase. 6-mercaptopurine inhibits the committed step of purine synthesis by blocking aminotransferase. OMP carboxylase can have a decreased expression linked with hereditary orotic aciduria, lowering UMP synthesis.
Macromolecules (Nucleic Acids).
- Which of the following statements about nucleic acid metabolism is incorrect?
- UMP is formed through the removal of CO2 from orotidine monophosphate
- The first step of pyrimidine nucleotide degradation is the conversion from nucleosides to nucleotides
- The controlled step of pyrimidine synthesis is the formation of carbamoyl phosphate by CPS-II
- Uric acid is the end product of purine degradation
- GMP synthesis is ATP-dependentAnswer B.
Macromolecules (Nucleic Acids).
PRPP is involved in all of the following processes, EXCEPT:
- Purine Synthesis
- Purine Degradation
- Pyrimidine Synthesis
- Purine Salvage Pathway
- None of the aboveAnswer B.
Macromolecules (Nucleic Acids).
Methotrexate will inhibit all of the following EXCEPT?
- Production of dTDP
- Production of UMP
- Production of IMP
- SAM production
- All of these choices are inhibited by methotrexateAnswer B.
Macromolecules (Nucleic Acids).
PRPP is an allosteric regulator for which of the following enzymes?
- CPS II
- Orotate phosphoribosyl transferase
- Glutamine phosphoribosyl amidotransferase
- A & B
- A & CAnswer E.
Macromolecules (Nucleic Acids).
Identify the following compound:

- Arachidonic Acid
- Linolenic Acid
- Stearic Acid
- Palmitic Acid
- Oleic AcidAnswer A. Among the following, only Arachidonic acid is omega-6 fatty acid.
Macromolecules (Lipids and Lipid Metabolism).
Which one of the following lipoprotein particles has highest concentration of triacylglycerol?
- The high-density lipoproteins (HDL)
- The chylomicrons (CM)
- The low-density lipoproteins (LDL)
- The very low-density lipoproteins (VLDL)
- Serum albumin-associated lipidAnswer B.
Macromolecules (Lipids and Lipid Metabolism)
Phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophylic substance. Where does each phospholipase C hydrolyze?

- A
- B
- C
- DAnswer B.
Macromolecules (Lipids and Lipid Metabolism).
When moving forward to inducing labor in one of your patients, what would be your best choice for relaxing the walls of the cervix and inducing uterine contractions?
- PGE2
- PGD2
- 5LOX inhibitor
- Non-steroidal anti-inflammatory drugs
- Selective COX2 inhibitor (Celebrex)Answer A.
Macromolecules (Lipids and Lipid Metabolism)
Essential fatty acid (EFA) deficiency is rare, occurring most often in infants fed diets deficient in EFAs. Signs include scaly dermatitis, alopecia, thrombocytopenia, and, in children, intellectual disability. Which of the following Fatty Acids would an individual with this disease be missing from their diet?
- Linoleic Acid
- Arachadonic Acid
- α-linolenic acid
- A & B
- A & CAnswer E.
Macromolecules (Lipids and Lipid Metabolism)
Which of the following correctly organizes Lipoproteins by their size?
- HDL > LDL > vLDL > Chylomicron
- Chyomicron > HDL > LDL > vLDL
- Chylomicron > vLDL > LDL > HDL
- vLDL > LDL > HDL > Chylomicron
- LDL > vLDL > HDL > ChylomicronAnswer C.
Macromolecules (Lipids and Lipid Metabolism).
The natural way of modulating eicosanoids is via controlling dietary intake. As a primary care physician, you inform a patient about the benefits of Mediterranean diet in the prevention of CVD. The Mediterranean diet is clinically tested to have higher concentration of omega 3 fatty acids. Which of the following fatty acids is part of Mediterranean diet?
- Arachidonic Acid
- α-Linolenic Acid
- Linoleic Acid
- Palmitic Acid
- Oleic AcidAnswer B.
Macromolecules (Lipids and Lipid Metabolism).
Which of the following is INCORRECT regarding Chylomicrons?
- Chylomicrons initially travel through the lymphatic system before being release into circulation via the Subclavian vein
- Chylomicrons are responsible for the transport of endogenous fatty acids
- Apo B-48 are markers for Chylomicrons
- They are mostly composed of TAGs
- Apo C-II are donated to Chylomicrons by HDL and are responsible for the activation of LPLAnswer B.
Macromolecules (Lipids and Lipid Metabolism).
Pravastatin is a member of the drug class of statins, used in combination with diet, exercise, and weight loss for lowering cholesterol and preventing cardiovascular disease. Which of the following best explains the mechanism of action behind this drug?
- Pravastatin inhibits the transfer of cholesterol to vLDL by inhibiting CETP
- Pravastatin is a competitive inhibitor of HMG-CoA Reductase
- Pravastatin deactivates Apoprotein AII
- Pravastatin deactivates Apoprotein AI
- None of the aboveAnswer B. In the lecture Enzyme and Enzyme Kinetics II, Pravastatin was used as an example for a competitive inhibitor. Its mechanism of action was given.
Macromolecules (Lipids and Lipid Metabolism).
It is often recommended for patients to take a daily dose of Aspirin if they are believed to be at risk for heart attack. This is because aspirin works to inhibit platelets and therefore can prevent blockages in the coronary arteries. Which of the following best describes the mechanism of action for Aspirin pertaining to its ability to prevent heart attacks?
- Aspirin inhibits COX-2 preventing the formation of Thromboxane A2
- Aspirin inhibits COX-1 preventing the formation of Thromboxane A2
- Aspirin inhibits COX-2 preventing the formation of Prostaglandin
- Aspirin enhances the action of COX-1, causing an increase in Prostacyclin
- Aspirin enhances the action of COX-2, causing an increase in ProstacyclinAnswer B. Aspirin works by inhibiting both COX-1 and COX-2, therefore we can eliminate answers D&E. The question is pertaining to Aspirin’s ability to prevent clotting. Since we know that COX-2 is responsible for the production of Prostacyclin (inhibits the aggregation of platelets) and Prostaglandin (produces inflammatory response), answers A & C can also be eliminated. This leaves only B which is the correct response.
Macromolecules (Lipids and Lipid Metabolism).
Which one of the following statements is CORRECT about the endogenous pathway of lipoprotein metabolism?
- LDL is derived from HDL
- Apolipoprotein B-48 mediates the binding to the LPL receptor
- Apolipoprotein E has no role in this pathway
- LDL is formed from vLDL in plasma
- The pathway begins with the secretion of the intestinal lipoproteinsAnswer D. VDLD and LDL are transporters for endogenous cholesterol and lipids from liver to tissue. LDL is derived form circulating VLDL after lipase depletes core and surface components. Apolipoprotein E is associated with VLDL. Apolipoprotein B-48 is associated with chylomicron.
Macromolecules (Lipids and Lipid Metabolism).
The enzyme that regulates the synthesis of fatty acid….
- Is located in the mitochondrial matrix
- Is involved in synthesis of ketone bodies
- Would have increased activity due to high levels of ADP
- contains a pair of phosphocreatine groups that are essential for its activity
- is expressed at different levels depending upon the composition of the dietAnswer E. acetyl CoA carboxylase, is in the cytoplasm. Diet with high/low carbohydrate will increase/decrease expression of acetyl CoA carboxylase. This action is mediated by insulin acting on transcriptional regulator.
Macromolecules (Lipids and Lipid Metabolism).
Which one of the following lipoprotein particles has highest concentration of cholesterol?
- The high density lipoproteins (HDL)
- The chylomicrons (CM)
- The low density lipoproteins (LDL)
- The very low density lipoproteins (VLDL)
- Serum albumin-associated lipidAnswer C.
Macromolecules (Lipids and Lipid Metabolism).
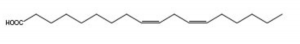
- Which of the following answer choices is true regarding the fatty acid shown above?

- It is an omega-6 fatty acid
- It is an omega-3 fatty acid
- It is a Cis isomer
- A & C
- B & CAnswer D.
Macromolecules (Lipids and Lipid Metabolism).
Patients with irritable bowel syndrome (IBS) are discouraged from taking NSAIDS such as aspirin, ibuprofen, and naproxen. Which of the following best explains the reasoning behind this?
- These NSAIDS are nonselective and can lower stomach prostaglandin levels
- These NSAIDS are COX-1 selective and can lower stomach prostaglandin levels
- NSAIDS lead to dangerously low levels of arachidonic acid
- Inhibition of cyclooxygenase initiates metabolic deficiencies due to low levels of leukotrienes
- These NSAIDS are nonselective COX inhibitors and can inhibit paracrine signaling factors in the epithelial cells of the small intestineAnswer A.
Macromolecules (Lipids and Lipid Metabolism).
- Acetyl CoA Carboxylase (ACC) is recognized as the committed step in the reductive pathway of fatty acid metabolism. While running in a marathon race, you would expect the activity of ACC to:
- Increase due to high levels of citrate
- Decrease due to high levels of long-chain fatty acids
- Increases due to high levels of Biotin
- A & B
- A, B & CAnswer D.
Macromolecules (Lipids and Lipid Metabolism).
- Fish oil is often recommended as a supplement to decrease inflammation in patients with arthritis. Which of the following best describes its role in the anti-inflammatory process?
- Fish oil has a high ratio of omega-3 fatty acids that are metabolized to produce prostaglandins with an anti-inflammatory effect
- Fish oil has a high ratio of omega-6 fatty acids that are metabolized to produce prostaglandins with an anti-inflammatory effect
- Fish oil has a high ratio of omega-3 fatty acids that inhibit the formation of prostaglandins with an inflammatory effect
- A & C
- B & CAnswer D.
Macromolecules (Lipids and Lipid Metabolism)
- Over your recent three-day weekend (extra study time), your friend in undergrad asks you why Na+ can’t move through a K+ channel even though Na+ is smaller. The best answer is:
- K+ binds to the four COO– ends of the channel proteins with higher affinity.
- The channel “tightens” in the presence of Na+ or H+, and only allows K+ through.
- H2O binds Na+ more tightly than K+.
- Selectivity filters can’t strip all the H2O molecules off Na+, but can remove all H2O from K+.
- Na+ doesn’t form H2O complexes, while K+ does. Answer D. Four H2O molecules form a complex around Na+ and K+. However, the four P segments are spaced so that they can only remove all four H2O molecules from K+. They are too far apart to pull all four H2O molecules off of Na+. Thus K+ is free to move through the channel while Na+ generally remains in the H2O complex.
Biomembranes and Membrane Transport.
Which of the following is NOT true regarding membrane fluidity?
- Cholesterol increases the fluidity of the membrane at low temperatures
- Cholesterol decreases the fluidity of the membrane at high temperatures
- Cis formation of unsaturated fatty acids increases the fluidity of the membrane
- Unsaturated fatty acids decrease the fluidity of the membrane
- Penguins will likely have more unsaturated fatty acids to compensate for lower temperaturesAnswer D. Unsaturated fatty acids increase the fluidity of the membrane.
Biomembranes & Membrane Transport.
Which of the following compounds is most likely to diffuse across a cell membrane via simple diffusion?
- Ethanol
- H2O
- Glucose
- CO2
- Na+Answer D.
Biomembranes & Membrane Transport.
Which of the following is NOT true regarding polyphosphoinositide signaling?
- G protein becomes activated in response to a hormone
- Phospholipase B cleaves Phosphatidyl Inositol releasing IP3 into the cytosol
- IP3 binds to a receptor on the ER causing a release of Ca2+ into the cytosol
- Ca2+ activates protein kinase CAnswer B. Phospholipase C cleaves Phosphatidyl Inositol releasing IP3 into the cytosol.
Biomembranes and Membrane Transport.
Which of the following is INCORRECT regarding the lipid membrane?
- The membrane is approximately 50% proteins in mass
- Sugars tend to face the extracellular space
- The outer membrane consists of more positively charged head groups
- The inner membrane has a large concentration of serine heads
- The inner membrane is reducing while the outer membrane in oxidizingAnswer C.
Biomembranes and Membrane Transport.
Which of the following is an example of primary active transport?
- GLUT1 Transporter
- Na+/K+ Pump
- SGLT1
- Na, Ca++ exchangeAnswer B.
A – Facilitated diffusion
C – Symport (Secondary Active transport)
D – Antiport (Secondary Active transport)
Biomembranes and Membrane Transport.
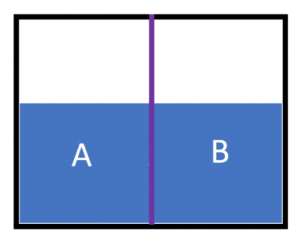
A container is separated by a semipermeable membrane (represented by the purple line). The picture below shows this container with equal amounts of distilled water on both side A and B. What would occur if a solution of glucose was added to side B?

- Water would diffuse through the membrane causing side A to rise
- Water would diffuse through the membrane causing side B to rise
- Glucose would cross the membrane and cause the concentration to be equal on both sides
- Glucose would cross the membrane causing the concentration to be greatest on side A
- The membrane prevents the movement of water and glucose, so the water level wouldn’t change on either side Answer B.
Biomembranes and Membrane Transport.
Choline is an important component in which of the following?
- Neurotransmitters
- Glycerol Phospholipids
- TAGs
- A & B
- A, B & CAnswer D. Choline is vital for Ach (neurotransmitter) and Glycerol phospholipids. TAGs do not contain choline. Biomembranes and Membrane Transport.
Which of the following molecules diffuse most rapidly across lipid bilayers?
- Na+
- K+
- H2O
- O2
- GlucoseAnswer D. Oxygen diffuses most quickly because it is non-polar, small, and non-charged.
Biomembranes and Membrane Transport.
Kartagener syndrome is a rare, ciliopathic, autosomal recessive genetic disorder that causes defects in the action of cilia and flagella. This condition is most likely associated with which part of the cytoskeleton?
- Microfilaments
- Microtubules
- Intermediate filaments
- Macrofilaments
- MacrotubulesAnswer B.
Biomembranes and Membrane Transport.
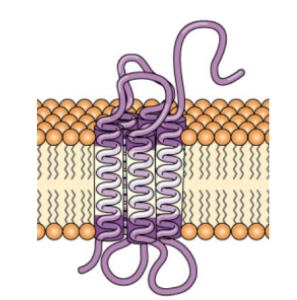
Which subtype of transmembrane protein is shown below?

- Type I
- Type II
- Type III
- Type IV
- Type VAnswer C.
Biomembranes and Membrane Transport.
The calcium ion is an important second messenger that is involved in many cellular processes. It is able to work as a second messenger since the cytosolic concentration is kept so low, allowing small increases in Ca2+ to have meaningful effects. Which of the following describes the type of signaling process that calcium displays?
- Intracrine
- Autocrine
- Paracrine
- Endocrine
- Juxtracrine Answer A. Intracrine signals are produced by the target cell, that stay within the target cell. Second messengers were cited as examples for this signaling type.
Cell Signaling.
Caffeine, a methylxanthine, has been added to a variety of cell types. Which one of the following would be expected in various cell types treated with caffeine and epinephrine?
- Decreased activity of liver protein kinase A
- Decreased activity of muscle protein kinase A
- Increased activity of liver pyruvate kinase
- Decreased activity of liver glycogen synthase
- Decreased activity of liver glycogen phosphorylation Answer D. Caffeine works by inhibiting phosphodiesterase and increasing the ½ life of cAMP. PKA (Protein Kinase A) is directly activated by cAMP and works to phosphorylate glycogen synthase (deactivating it) and phosphorylase kinase (activating it). Remember that Viagra also works by inhibiting phosphodiesterase, but instead it causes an increase in the ½ life of cGMP which leads to smooth muscle relaxation (vasodilation) to cause an erection.
Cell Signaling.
GEFs are a potential target for cancer therapy due to their role in many signaling pathways, particularly cell proliferation. A chemotherapeutic drug that targets GEF would work by…
- Preventing the transphosphorylation of receptor tyrosine kinase dimers
- Slowing the process involved in the activation of the G protein alpha-subunit
- Preventing the dimerization of receptor tyrosine kinase subunits
- Directly preventing the release of cAMP and, therefore, the activation of protein kinase A
- Preventing the binding of the ligand to the G-protein-coupled receptor (GPCR) Answer B. GEF is responsible for the exchange of GDP for GTP on the α-subunit, which in turn leads to a conformational change and dissociation of the Gα from the receptor and the Gβγ subunits. GAP, in contrast, speeds up the hydrolysis of GTP → GDP, deactivating the α-subunit.
Cell Signaling.
You are on rotations in a primary care clinic when a resident asks you to give an injection of Humira (Adalimumab) to a patient with Rheumatoid arthritis. However, this is the patient’s first injection so the resident would also like you to explain the mechanism by which this drug works before administering it. How are you going to explain this to your patient?
- Humira works as a competitive inhibitor for the TNF-α receptor, preventing the inflammatory response associated with their condition
- Humira works by binding directly to TNF-α, preventing the inflammatory response associated with their condition
- Humira works by binding to NF-kB, preventing the inflammatory response associated with their condition
- Humira works by binding to IkB, preventing the inflammatory response associated with their condition
- Humira works by promoting apoptosis of leukocytes, preventing the inflammatory response associated with their condition Answer B. Humira is a monoclonal antibody and works against TNF-α to prevent the inflammatory response associated with RA. Etanercept is a soluble receptor that binds to TNF-α.
Cell Signaling.
Herceptin is a monoclonal antibody that has been shown to be extremely effective in treating breast cancer that is HER2 receptor positive.Which of the following explains the mechanism of action by which Herceptin works?
- Herceptin is a soluble receptor that binds to human epidermal growth factor to prevent its binding with the HER-2 receptor
- Herceptin works by binding directly to the HER-2 receptor in order to tag the cell for destruction
- Herceptin work by binding to the HER-2 receptor, inhibiting it, and thus preventing cell signalling
- A & B
- B & CAnswer E.
Cell Signaling.
You decide to partake in an exciting research opportunity during the summer before your 2nd year. You are told that the research will be regarding a newly discovered disease called Diabetes Imaginarius, which resembles Diabetes Mellitus. In individuals with this disorder, IP3 gated calcium channels are fully functional, but are produced much less frequently than needed. Therefore, there is an inadequate release of calcium from the endoplasmic reticulum and a stunted response to insulin. Which of the following best describes this newly found disease?
- Genetic channelopathy
- Toxic Channelopathy
- Transcriptional channelopathy
- Autoimmune channelopathy
- None of the above (Answer C. Just in case you couldn’t tell, Diabetes Imaginarius is a completely made-up disorder. You can infer that it is a transcriptional channelopathy because the question states that the channels are fully functional, but are produced less frequently.
Cell Signaling.
Gleevec (imatinib) has shown to be an effective treatment for chronic myeloid leukemia. What action does this drug perform that leads to such positive therapeutic outcomes?
- Inhibiting the constitutive tyrosine kinase activity of the Abl protein subunit
- Inhibiting the constitutive tyrosine kinase activity of the Bcr protein subunit
- Preventing the chimeric fusion of the Bcr-Abl protein
- Upregulating production of Bcr-Abl protein subunits
- Using soluble a RTK analog to sequester pro-proliferation signaling moleculesAnswer A. The others are all simple distractors. B is incorrect because the tyrosine kinase activity is seen in the Abl subunit, not Bcr. Imatinib does not have an effect on the actual formation of the chimeric protein. Option E is the mechanism of Humira on the TNF-a signaling molecule.
Cell Signaling.
Receptor tyrosine kinases can be mutated in such a way that they lead to increased cell growth, and ultimately, cancer. Which of the following is NOT one of the recognized mutations leading to cancer development?
- A mutation resulting in native dimerization of the RTK subunits
- A mutation in RTK transcription resulting in too many membrane receptors
- A mutation in RTK transcription limiting the number of membrane receptors
- A mutations resulting in amino acid residue changes to the phosphorylation pocketAnswer C. All of these are true except for C. If RTK membrane receptor expression was decreased, there would be a lowered response in the cell proliferation pathway, and cells would not rapidly divide as seen in cancer.
Cell Signaling.
Approximately one third to one half of all pharmaceutical drugs work by what general mechanism?
- Disruption of G protein-coupled receptors
- Phospholipase C analogues
- PI3K deactivation
- Inhibition of Smad dephosphorylation
- Competitive inhibition of TRK homodimer substrate Answer A.
Cell Signaling.
Which of the following enzymes is activated by cAMP, passing on the hormonal signal?
- Protein kinase A.
- Protein kinase B.
- Protein kinase C.
- G protein receptor kinase
- PI 3-KinaseAnswer A.
Cell Signaling.
One of the insulin signal transduction pathways results in the activation of phosphatidylinositol 3′ kinase (PI 3-Kinase). Which of the following describes the following events after the activation of PI 3-Kinase?
- Protein kinase B, an active serine kinase, dissociates from the membrane
- Protein kinase B, an active JAK/STAT, dissociates from the membrane
- Protein kinase B, an active serine kinase, associates with the membrane (becomes tethered)
- Protein kinase A, an active tyrosine kinase, associates with the membrane (becomes tethered)
- Protein kinase A, an active serine kinase, associates with the membrane (becomes tethered) Answer A. Don’t forget that PKB is the same as Akt!
Cell Signaling.
- Which of the following is TRUE regarding insulin receptors?
- They are serine threonine kinase receptors
- They are dimerized upon binding to insulin
- They require the use of IP3 second messenger to cause Ca2+ release from the Golgi apparatus
- They activate PLC, which catalyzes the reaction of PIP2 into DAG and PIP3
- IRS provides docking sites for PI3-kinase, PLC, and Grb2Answer E.
Cell Signaling.
Anisha, a ravenous OMSI with a sweet tooth, is eating yet another snickers out of the goodie bags that were given out during national primary care week. After the consumption of this treat, her insulin levels rise in response to an elevated BGL. Which of the following is true regarding the insulin signaling pathway occurring in Anisha?
- Glycogenesis is occurring due to the phosphorylation of glycogen synthase by protein phosphatase
- Insulin is received by a serine-threonine kinase receptor
- Glycogenesis is occurring due to the dephosphorylation of glycogen phosphorylase by protein phosphatase
- Glycogenesis is occurring due to the dephosphorylation of glycogen synthase by PKB
- Activated IP 3-Kinase facilitates the reaction of PIP2 to PIP3Answer E. Activated IP 3-Kinase facilitates the reaction of PIP2 to PIP3
A: phosphorylation of glycogen synthase results in its deactivation
B: Insulin is received by a tyrosine kinase receptor
C: This answer choice should have read “Glycogenesis is occurring due to the phosphorylation of glycogen phosphorylase by protein phosphatase” Phosphorylation activates glycogen phosphorylase, causing glycogen degradation which is not response to insulin
D: This is true, except the phosphorylation occurs from protein phosphatase, not from PKB. PKB phosphorylates protein phosphatase.
Cell Signaling.
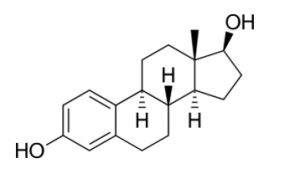
Estradiol is the major female sex hormone and is involved in the regulation of estrous and menstrual female reproductive cycles. Thus, it is used in birth control pills it in order to prevent pregnancy and control menses. The structure of estradiol is shown below. Based on your knowledge of cell signaling, which of the following receptor types does estradiol most likely utilize?

- Tyrosine Kinase Receptor
- JAK-STAT Receptor
- G-protein Coupled Receptor
- Nuclear Hormone Receptor
- Small G-protein Receptor Answer D. From the structure, you should be able to tell that estradiol is a cholesterol derived hormone. Hence, it is lipophilic and can travel through the lipid bilayer which would allow for it to bind to an intracellular receptor.
Cell Signaling.